
SPRAVATO® (esketamine) is an intranasal formulation of ketamine administered in a clinical setting under supervision, usually in combination with an oral antidepressant, for treatment-resistant depression or other conditions. Responsive Centers is a certified SPRAVATO® treatment center.
Looking for esketamine therapy?
-
Clients | Get started by submitting an Ketamine Therapy Request.
-
Referrals | Head over to our Referrals page to request ketamine therapy for someone in your care.
What is ketamine therapy?
SPRAVATO® (esketamine) is a breakthrough treatment option for individuals experiencing treatment-resistant depression or other complex mood disorders. This FDA-approved medication is a nasal spray form of esketamine, a derivative of ketamine, and is administered in a clinical setting under the supervision of trained medical professionals.
Unlike traditional oral antidepressants that can take weeks to begin working, SPRAVATO® may offer relief more rapidly by targeting glutamate, a different neurotransmitter system in the brain. It is typically used in combination with an oral antidepressant to enhance its effectiveness and support long-term symptom relief. Candidates for SPRAVATO® are often those who have not responded adequately to at least two other antidepressant treatments.
Treatment sessions take place in our office, where clients self-administer the nasal spray and are then monitored for approximately two hours by our clinical team for safety and side effects. During this time, we provide a calm, supportive environment to ensure comfort and stability throughout the experience. Follow-up frequency is determined based on each person’s response to treatment and is discussed collaboratively with the prescribing provider.
Ketamine therapy offers hope for those who have struggled to find relief through traditional means. While not right for everyone, it can be a powerful tool in the broader journey of healing. Our team conducts a thorough psychiatric evaluation to assess eligibility, answer questions, and guide each client through the process with care and compassion.

Rohit Saha, MD is a psychiatrist at Responsive Centers who is now accepting new client appointments to assess fit for SPRAVATO® (esketamine) ketamine therapy.
.jpg)
What is SPRAVATO® (esketamine)?
SPRAVATO® (esketamine) is a form of ketamine approved by the FDA for major depressive disorder and treatment-resistant depression. If you have tried two or more oral antidepressants and are still experiencing depression symptoms, you may have treatment-resistant depression.
SPRAVATO® is the first treatment of its kind available and accessible to adults who have not shown significant improvement from standalone first-line treatments, such as psychotherapy and oral antidepressants.
The nasal spray is self-administered under the supervision of a trained provider and taken alongside an oral antidepressant.
Unsure if SPRAVATO® is right for you?
View eligibility guidelines here.
SPRAVATO® at Responsive Centers
Rohit Saha, MD is booking initial consultations with clients to assess fit for SPRAVATO® treatment. If you are a good candidate for ketamine therapy or have been referred to receive services at Responsive Centers, get started today by requesting your initial consultation! Dr. Saha and his team will ask about past treatment history, explain the treatment process, answer the client’s questions, and review important safety information. If the client is determined to be a good fit, they can schedule an appointment to receive their first dose under supervision at Responsive Centers, a certified SPRAVATO® treatment center.
SPRAVATO® is taken twice a week for the first four weeks. After the first four weeks, the client and provider will discuss and determine if treatment should be continued and at what dosage and frequency of administration.
SPRAVATO® Dosing for Treatment-Resistant Depression
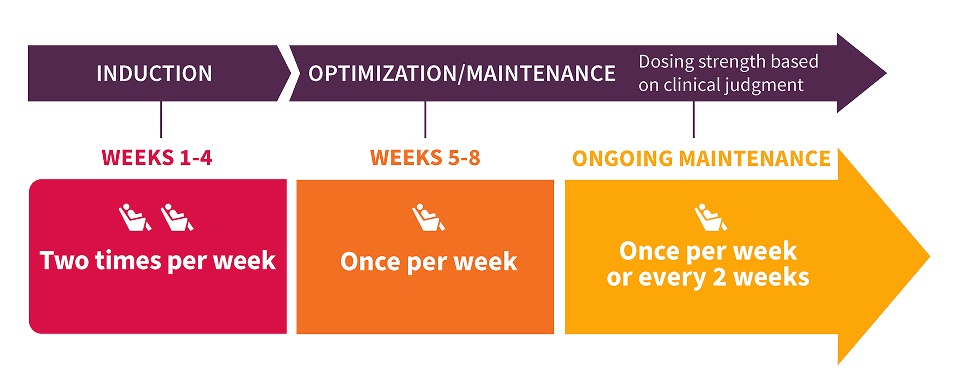
Note: SPRAVATO® REMS (Risk Evaluation and Mitigation Strategy) is a restricted program which requires close monitoring of safety because of the risks for sedation or loss of consciousness, dissociation, respiratory depression and abuse and misuse.
SPRAVATO® is only available through SPRAVATO® REMS and can only be administered at healthcare settings certified in the program. Clients treated in outpatient healthcare settings (e.g., medical offices and clinics) must be enrolled in the program.
Where can I learn more about SPRAVATO®?
The SPRAVATO® website may help answer additional questions about safety and tolerability, clinical studies, and more. Please note: This is not a substitute for professional medical advice.
Important Safety Information
(Updated 7/10/2024)
SPRAVATO® can cause serious side effects, including:
Sedation and dissociation. SPRAVATO® may cause sleepiness (sedation), fainting, dizziness, spinning sensation, anxiety, or feeling disconnected from yourself, your thoughts, feelings, space and time (dissociation).
- Tell your healthcare provider right away if you feel like you cannot stay awake or if you feel like you are going to pass out.
- Your healthcare provider must monitor you for serious side effects for at least 2 hours after taking SPRAVATO®. Your healthcare provider will decide when you are ready to leave the healthcare setting.
Respiratory depression was observed with the use of SPRAVATO®; additionally, there were rare reports of respiratory arrest.
- Your healthcare provider must monitor you for serious side effects for at least 2 hours (including pulse oximetry) after taking SPRAVATO®. Your healthcare provider will decide when you are ready to leave the healthcare setting.
Abuse and misuse. There is a risk for abuse and physical and psychological dependence with SPRAVATO® treatment. Your healthcare provider should check you for signs of abuse and dependence before and during treatment with SPRAVATO®.
- Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines, or street drugs.
- Your healthcare provider can tell you more about the differences between physical and psychological dependence and drug addiction.
Increased risk of suicidal thoughts and actions. Antidepressant medicines may increase suicidal thoughts and actions in some people 24 years of age and younger, especially within the first few months of treatment or when the dose is changed. SPRAVATO® is not for use in children.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a higher risk of having suicidal thoughts or actions. These include people who have (or have a family history of) depression or a history of suicidal thoughts or actions.
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behavior, thoughts, or feelings, or if you develop suicidal thoughts or actions.
- Tell your healthcare provider right away if you have any new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms.
Tell your healthcare provider right away if you or your family member have any of the following symptoms, especially if they are new, worse, or worry you:
- Suicide attempts
- Thoughts about suicide or dying
- Worsening depression
- Other unusual changes in behavior or mood

